Describe the Physical Properties of Table Salt
Table salt used universally as a seasoning is fine-grained and of high purity. Table salt is an ionic compound which breaks into its component ions or dissociates in water.

Classification Of Matter Ultimate Card Sort For Pure Substances And Mixtures Sorting Cards Teacher Preparation Mixtures
In Old English its sealt in Dutch zout German salz and in French of course selIts a word that is deceptively simple yet full of a rich and complex history both social and geologic.

. Boiling point 1413 degrees Celsius. Table salt may be white or may have a faint purple or blue tinge from impurities. Colorless or white when pure.
There are many different types of salt including pickling salt and kosher salt but table salt is the kind most used in recipes. Salt in English from its Indo-European roots is a stately combination of the Latin sal and the Greek hals. It is easily soluble in water and partially soluble or insoluble in other liquids.
Salt has been used throughout most of written history to flavor and preserve food. Describe one more that is based on your own experience with using table salt. The sodium and chlorine atoms are present in equal amounts 11 ratio arranged to form a cubic crystal lattice.
Beside density strength and hardness determine the physical properties of a saltmineral. Physical Properties of Halite. General Properties of Salts.
Some of the characteristic properties of salts are. When Chlorine is added to Sodium it takes on a more white clear colour. Slightly soluble in alcohol but insoluble in concentrated hydrochloric acid.
The Following Statments describe some physical and chemical properties of sucrose table sugar. The molecular formula of table saltsodium chlorideis NaCl. The tap water is colorless while the table salt is white in color.
Has no odour but strong taste. Table salt is 97 to 99 sodium chloride NaCl. Transparent and colourless in crystalline form rather like ice.
General Properties of Salts. HELP ME PLEASEEE Four properties of sodium chloride NaCl are listed below. Since 1972 in Japan table salt is produced by electrodialytic concentration of sea water.
It is salty-tasting and corrosive to base metals. Which response includes all that describe chemical properties and none that describe physical properties. Both refer to the cohesion properties that depend on the type and strength of the bonds in the crystal lattice and are thus subject to anisotropy.
To ensure that this hygroscopic ie water-attracting substance will remain free-flowing when exposed to the atmosphere small quantities of sodium aluminosilicate tricalcium phosphate. However other compounds are present in table salt depending on its source or additives that may be included before packaging. The density of the tap water is 1 gcm3 while that of table salt is 2165 gcm3.
Solid at a room temperature. Salts can be clear orange blue green or black in color. Salts are mostly solids which melt as well as boil at high temperatures.
Salt is a chemical compound with a number of interesting properties. It forms where large volumes of sea water or salty lake water evaporate from an arid-climate basin -- where there is a replenishing flow of salt water and a restricted input of other water. Melting and boiling points.
Impure salts are those that have lost their salty taste. General Properties of Salts. For example sodium chloride potassium sulphate aluminium nitrate ammonium carbonate etc are soluble salts while silver chloride.
Rock salt is the name of a sedimentary rock that consists almost entirely of halite a mineral composed of sodium chloride NaCl. An increase in the cross-linking density of a cation-exchange membrane or the formation of a thin. Common salt or sodium chloride is the chemical compound NaCl.
Physical Properties of NaCl Sodium chloride a white crystalline solid contains a density of 2165 gmL a melting point of 801 C and a boiling point is about 1413 C. Salts are crystals that have a very high melting point. The table salt has a boiling point of 1 413 degree Celsius while the boiling point of water is 100 degree Celsius.
It is also available as aqueous solutions with different concentrations which are known as saline solutions. For example when sodium chloride is heated with sulphuric acid sodium hydrogensulphate at low temperature and then sodium sulphate at high temperature are produced and hydrogen chloride gas is evolved. Reaction with an acid.
Sodium chloride is another name for table salt. The melting point of water is zero degree. Transparent and colourless in crystalline form rather like ice.
Perfect cubic three directions at right angles. When a salt reacts with an acid another salt and acid are formed. It chars or blackens when heated gently.
It is a colorless solid. Its density is 16 gmL. Crystals or white crystalline powder.
Pure salt is completely free of odor. While common table salt is sodium chloride NaCl salts can more broadly be defined as the product formed from neutralizing an acid where a metal atom or positively charged radical replaces one or more of the acids hydrogen atoms. Is course to the touch when in small grains.
Impurities produce any color but usually yellow gray black brown red. Is clear when pure although may also appear white grey or brownish depending upon purity. 2Nas Cl 2 g 2NaCls Properties of Sodium Chloride.
Soluble in water 356g100g at 0C and 392g100g at. The pH of sodium chloride ranges from 67 to 73. Soluble in water 356g100g at 0C and 392g100g at 100.
Melting point 801 degrees Celsius. Crystallises in the isometric system usually in the form of cubes. Pure sodium chloride is an ionic crystal solid.
These ions are Na and Cl-. For the specific requirements of this process ion-exchange membranes have been developed which can separate to a large extent monovalent ions from a solution mixed with multivalent ions. However sodium and chlorine respond together to generate a substance that is familiar to nearly everybody in the globe that is sodium chloride or table salt or common salt.
The tap water is a liquid while the salt is a solid at room temperature. Commonly known as table salt it is a white-colored or transparent water-soluble crystalline solid with the chemical symbol NaCl. It can be sweet savory bitter salty or sour in taste.
Salt is a compound NaCl made up of two elements and table salt contains some additional ingredients. Crystallises in the isometric system usually in the form of cubes. Sodium chloride boils at 1465 degrees Celsius and melts at 801 degrees Celsius.
Salts are generally soluble in water. In its pure form sodium chloride is white. Salts are thus neutral ionic compounds composed of positively charged ions cations.

Himalayan Salt Magic Herbs Herbal Magic Herbalism

What Are Salts Class 10 In Urdu Hindi Acids Bases And Salts Chemistry The Creator

Salt Chemistry History Occurrence Manufacture Uses Facts Britannica

Physcial And Chemical Properties Of Sodium Chloride Download Scientific Diagram

Fine Pink Crystal Himalayan Sea Salt 84 Minerals Fda Kosher Gourmet Pure Organic 846836007727 Ebay Himalayan Pink Salt Pink Salt Pink Salt Benefits

Lesson Chemical And Physical Changes Lab Stations Chemical And Physical Changes Physical Change Physics

Table Salt An Overview Sciencedirect Topics

More About Salts Properties Types With Solved Examples And Videos

Physical Chemical And Biological Properties Of Water Chemical Physics Property

Describe The Preparation Of Soluble And Insoluble Salts Preparation Salt Sodium Hydroxide

Lesson Chemical And Physical Changes Lab Stations Chemical And Physical Changes Physical Vs Chemical Properties Chemical Changes

Aquaponic Nutrients To Use Instead Of Salt Periodic Table Of The Elements Periodic Table Chemistry Periodic Table

Physcial And Chemical Properties Of Sodium Chloride Download Scientific Diagram
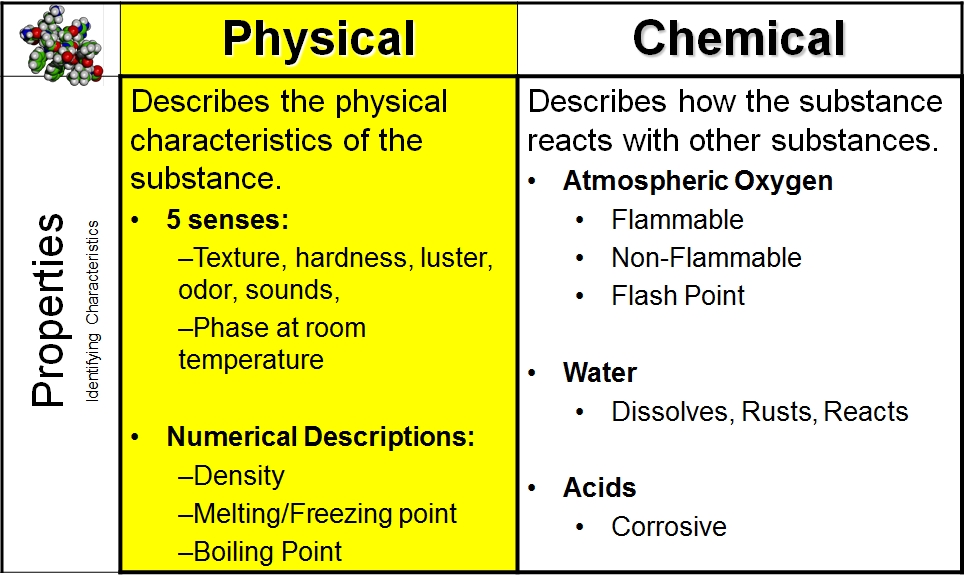
Chemical Physical Properties Vista Heights 8th Grade Science

Physical Properties Of Water Science Worksheets Physics Physical Properties

How Is Salt Made Lesson For Kids Video Lesson Transcript Study Com

The Nature Of Matter The Properties Of Matter What Is Matter Easier To Describe Than To Define It Is The Stuf Properties Of Matter Matter Unit Define Matter

Periodic Properties Lab Determine Periodic Trends From Lab Data Chemistry Classroom Chemistry For Kids Chemistry

In This Picture It Shows The Atomic Number Electron Configuration Atomic Mass And The Symbol Nirvana Songs Periodic Chart Songs
Comments
Post a Comment